|
|
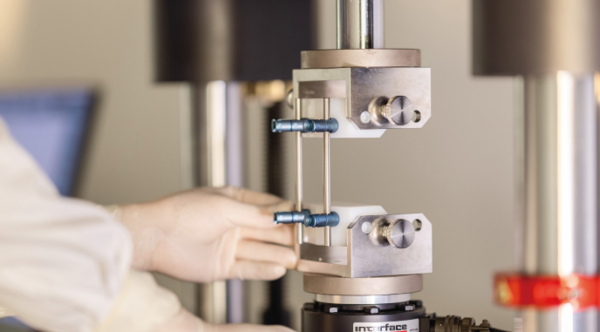
Human Factor / Usability Evaluation Manufacturers should follow human factors or usability engineering processes during the development of new medical devices, focusingspecifically on the user interface, where the user interface includes all points of interaction between the product and the user(s) including elements such as displays, controls, packaging, product labels, instructions for use, etc. Human factors / Usability validation testing is conducted to demonstrate that the device can be used by the intended users without serious useerrors or problems, for the intended uses and under the expected use conditions. Our Services: Establish Validation Test Protocol. Perform validation test in simulated clinical application environments. Complete Human Factor / Usability Evaluation Report.
|